Most of the Elements in Groups 16-18 Are Classified as
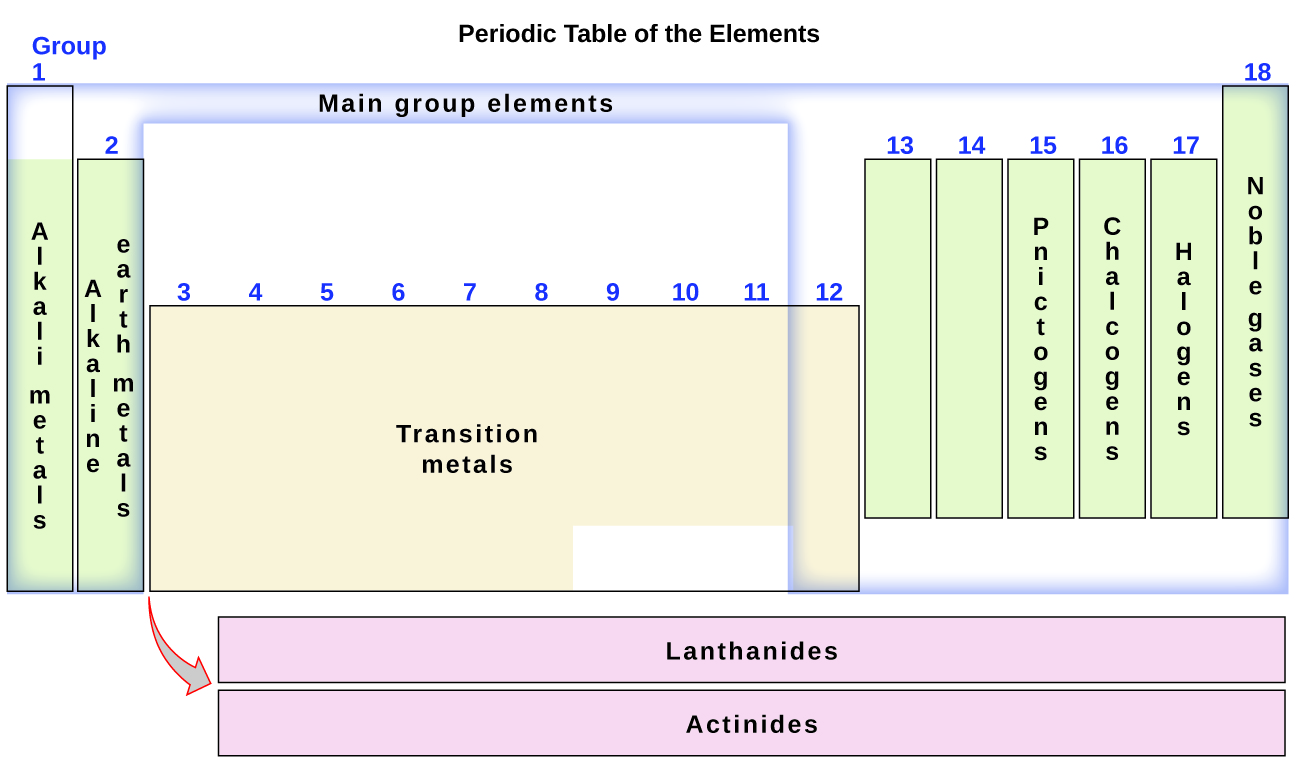
Alkali metals b. Iii E has a smaller atomic radius than D because E is on the right side of the modern periodic table.
2 5 The Periodic Table Chemistry
1 2 and 13 2.

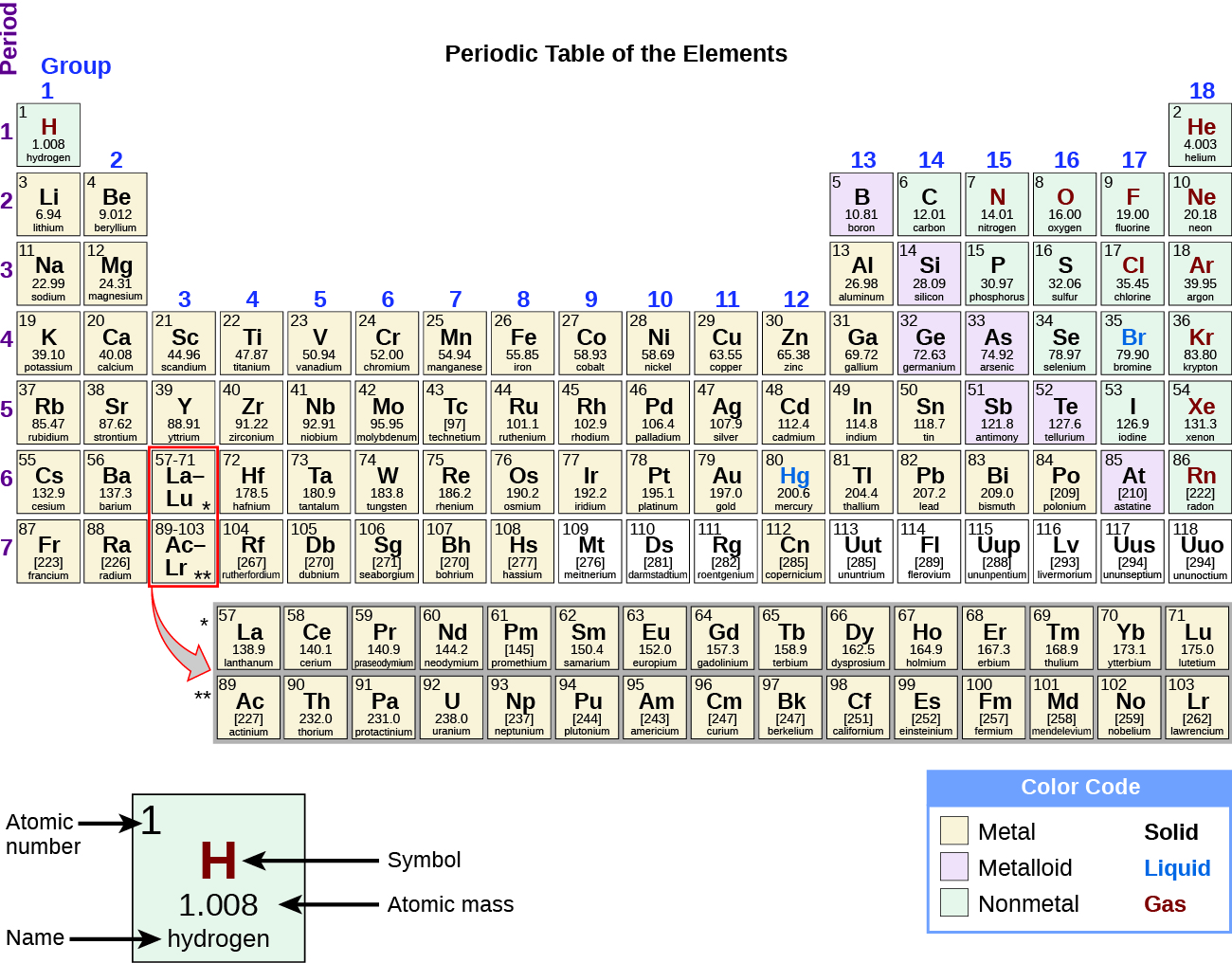
. This means that these elements are chemically inert and do not take part in any reaction. The main group elements of the periodic table are those elements that belong to the s and p blocks. The 4 valence electrons says that it is located in group 4A or group 14 in modern notation.
Alkaline earth metals most of the elements in groups 6A-8A are classified as. Group one has seven known elements the most of any of the 18 groups on the periodic table so far. Inner transition metals c.
Hence this element is carbonThe element has 2 electron shells means the element is in the 2nd group. Elements are typically classified as either a metal or nonmetal but the dividing line between the two is fuzzy. Groups 1 and 2 13 through 18.
The element Oxygen 159994. 14 15 and 16. View the answer now.
The group sixteen elements react with hydrogen to form hydrides of the sort H 2 E where E could be any element- oxygen sulfur selenium tellurium or polonium. In chemistry and atomic physics the main group is the group of elements sometimes referred to as representative elements whose lightest members are represented by helium lithium beryllium boron carbon nitrogen oxygen and fluorine as. Identify the period and group of the element.
Aubameyang14 Aubameyang14 The four elements that are most likely in a group is Titanium Ti Zirconium Zr Hafnium Hf and Rutherfordium Rf. On the Periodic Table it is found as O. Add your answer and earn points.
Number of electrons an atom needs in its outer level to be most stable. Most elements are metals. Which energy level of the period 4 transition elements is being filled with electrons.
Dry air contains 20946 oxygen by volume. These are the groups 13 to 17. The Group 18 elements include Helium He Neon Ne Argon Ar Krypton Kr Xenon Xe and Radon Rn.
Most elements in groups 16 through 18 are classified as. This is sometimes known as a group of chalcogens. Which three groups of the Periodic Table contain the most elements classified as metalloids semimetals.
The alkali metals make up most of Group 1 the tables. Group 18 elements. Most of the elements in groups 16-18 are classified as.
They are referred to as noble gases or inert gases. Across the period atomic sizeradius decreases on moving left to. Radii as you move from left to right across a period.
These four elements are most likely in group 1 See answer Advertisement Advertisement evanthegreat is waiting for your help. Oxygen is the most abundant of all the elements on the earth. The Physical States of Hydrides of Group 16 Elements.
The elements in Group 2 are classified as1 metals 3 nonmetals 2 metalloids 4 noble gases was asked on May 31 2017. The trend in the atmic radii as you move down the group 1 elements is due to. Group 18 Noble gasses are least reactive due zero valency of group 18 elements.
From the metalloids group 14 has five metalloids Carbon Silicon Germanium Tin and Lead group 15 has four metalloids Phosphorus Arsenic Antimony and Bismuth and group 16 has four metalloids Sulfur Selenium Tellurium and Polonium. The elements in this group are also known as the chalcogens or the ore-forming elements because many elements can be extracted from the sulphide or oxide ores. Shielding by inner electrons.
The regular oxidation states showed by the elements of group 16 incorporate -2 2 4 and 6. A K B B C I D S 39Which element is a member of the halogen family. Statement that when the elements are arranged by increasing atomic number there is a periodic repitition of their chemical and physical properties.
A bromine B argon. Trned in atomic radii as you move down the group 1 elements is partially due to. Oxygen forms about 466 by mass of earths crust.
2 13 and 14 3. These four elements are most likely in group 15. Wha is the trends in atomic radii as you move from left to right across a period.
37The elements in Period 5 on the Periodic Table are arranged from left to right in order of A 1 2 and 13 B 2 13 and 14 C 14 15 and 16 D 16 17 and 18 38Which three groups of the Periodic Table contain the most elements classified as metalloids semimetals. Ii C is the least reactive element. Oxygen sulphur selenium tellurium and polonium constitute Group 16 of the periodic table.
The group 16 elements of modern periodic table consist of 5 elements oxygen sulphur selenium tellurium and polonium. Groups 3 through 12. Because it belongs to group 18.
Counting the columns or table groups across the table ignoring the transition elements gives 8 element groups which match the filling of the eight spaces for electrons in the ns and np subshells n s 2 n p 6The s subshell can hold two electrons while the p subshell can hold. Shielding by inner electrons. In fact so many elements are metals there are different groups of metals such as alkali metals alkaline earths and transition metals.
Hopefully this helps. Most of the elements in groups 6A-8A are classified as a. Most metals are shiny solids with high melting points and densities.
What three groups of the periodic table contain the most elements classified as metalloids. A column in the periodic table.
2 5 The Periodic Table Chemistry

Electronegativity Chart 4 Element Chart Periodic Table Chart

Periodic Table Placemat Chemistry Activities How To Memorize Things Element Chemistry
No comments for "Most of the Elements in Groups 16-18 Are Classified as"
Post a Comment